MEDICAL DEVICES

LABORATORY TESTS
Lab4LIFE offers a wide range of microbiological and physic-chemical tests for pharmaceutical and medical device industry:
- Bioburden test;
- Sterility test;
- LAL test;
- Challenge test according to Ph. Eur.;
- Microbiological analysis on pharmaceutical according to Ph. Eur.;
- Evaluation of efficacy for disinfectants;
- Sterilization and washing process validation;
- Stability and shelf life evaluation using accelerated aging tests with ICH climate chambers;
- Clean room validation;
- EO residuals;
- Packaging validation;
- Global and specific migrations;
- Materials characterization according to ISO 10993-18;
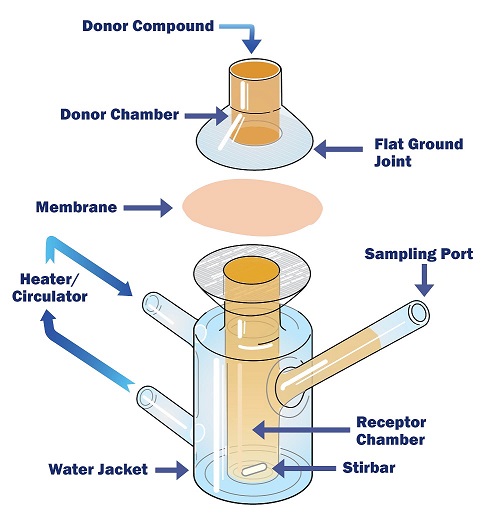
- Formulations for cutaneous / nasal / buccal use - In vitro permeation test in Franz cell for the evaluation of the functionality of medical devices based on substances, useful for their classification according to REGULATION (EU) 2017/745
BIOCOMPATIBILITY TESTS
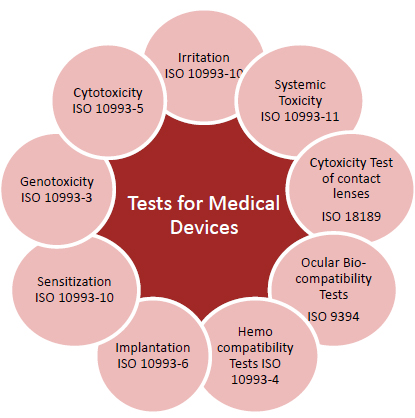
In partnership with a fully compliant to GLP and ISO 17025 test facility we can offer the complete list of biocompatibility tests according to ISO 10993 standards.
- Cytotoxicity according to ISO 10993-5;
- In vitro and in vivo skin irritation according to ISO 10993-10;
- In vivo skin sensitization according to ISO 10993-10;
- Haemocompatibility tests according to ISO 10993-4;
- Subcutaneous / muscular / bone implant according to ISO 10993-6;
- Genotoxicity tests according to ISO 10993-3;
- Subchronic toxicity according to ISO 10993-11;
- Materials characterization tests according to ISO 10993-18;
CONSULTANCY
Our team has built up specific competence in the regulatory framework of medical device class I, IIa IIb; III. We can support our customers in resolving issues related to regulatory compliance and assessment of the safety and effectiveness and process.
Main consultancy services:
- Support for the design of the biological risk assessment of medical devices and the planning of the best testing strategy based on the new requirements of ISO 10993-1:2018;
- Toxicological evaluation of data obtained from chemical characterization tests of components of medical devices;
- Planning the best testing strategy for the pre-clinical assessment of medical devices for dentistry use according to ISO 7405;
- Design of Study Protocols according to ISO 10993 series;
- Design of operating protocols for sterilization process;
- Design of operating protocols for washing process;
- Design of Scientific rationale for the biocompatibility assessment of medical devices;
- Evaluation of toxicological data to support product efficacy and safety;
